Lab 3: 9/6/18
Objective of this lab:
There were multiple objectives for this lab. The first 2 objectives were to be able to understand how a compound microscope works and to understand where ciliates are on the tree of life. After this lab we should also be able to make a wet prep and focus using 4x, 10x and 40x and be able to use methyl cellulose and stains to better observe the specimens.
Purpose of this lab:
The purpose of this lab was to correctly utilize a compound microscope so that we are able to see the ciliates in more detail. In addition we will be able to use our Field of View to estimate the size of our specimens.
Procedure:
- Uncover compound microscope and using a plate with Lycopodium Strobilus, go over how to focus the microscope correctly. Doing this we make sure to focus using the coarse adjustment only on 4x.
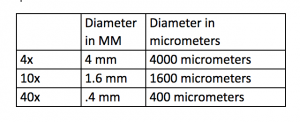
- Next we use the millimeter side of a ruler to measure our field of view and we use the equation FOV (low) x Mag (low) = FOV (high) x Mag (high). We are also sure to convert to micrometers.
- Using a dissecting scope we choose one of six unknown specimens and place a drop onto a concave plate so the ciliates are able to move around. This is the wet mount.
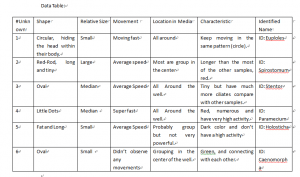
- Place wet mount under compound microscope and focus it. Record data. Recording should include measurement of specimen, color, movement and pictures.
- Do the same for 10x and 40x.
- Remove wet mount and set aside concave plate.
- Using a flat plate take a drop of same specimen and add a drop of methyl celluose. Record data at 4x, 10x and 40x again.
- Using a flat plate take 2 drops of specimen. Place one drop on each side of the plate. On one drop place methyl green pronin Y. On the other drop place Lugol’s Iodine. Record date at 4x, 10x and 40x from both drops.
- Clean off plates with bleach in sink and leave them to dry.
- Store compound scope properly.
Observations:
Observing my specimen on the wet mount at 4x magnification, I found the specimens were small and had a reddish tint to them. With my field of view at a diameter of 4mm (diameter in micrometers was 4000) I estimated around 20 ciliates could fit across. There was a 200 measurement. At 10x the diameter for my field of view was 1.6 mm (1600 micrometers). The measurement was 80. Still using the wet mount my specimen at 10x still had a reddish tint. Through 10x I was able to see the patterns of the ciliates more clear. Some ciliates seemed to be moving and other didn’t. If they were moving they were very slow. At 40x my field of view was .4mm (400 micrometers). The measurement was 20. This was the last magnification using the wet mount. In 4ox only one ciliate was seen and it was moving so slow it looked as if it was barely moving. Next I used a flat plate and added a drop of methyl cellulose. Methyl cellulose is supposed to slow down the ciliates. My first drops of specimen and methyl cellulose did not pick up anything. I re-tried and could only find one organism. The ciliate was not moving at all and the measurement was 200. At 10x and 40x I could not pick up on anything other than the one ciliate. It was still not moving. Next using a flate plate I put one drop of specimen and one drop of Methyl green Pronin Y. This is supposed to stain the ciliates. With this you have to observe quickly because the cells will begin to die off. At 4x for this one I could identify one ciliate. It was not moving at all. At 10x I could see the same ciliate in more detail. At this point it appeared a tinted color because of the Methyl Pronin Y. At 40x the ciliate was much more clear and more details became available. You could somewhat see the texture of the outer part of the ciliate as well as parts of the inside. Some organelles also became into view although it was not completely clear. Next I used Lugol’s Iodine. This is supposed to shrink the cell size. At 4x I identified one ciliate and the Iodine caused it to have a orange-yellow tint. The ciliate was around 2 mm long (2000 micrometers). The ciliate was completely still. At 10x the ciliate was still completely still but the image was more clear. The color of the dye tinted the color of the ciliate making it less opaque. At 40x I could not locate the ciliate and the glass was tinted yellow.
Storage: Glass Plates are washed with bleach and left out to dry. The compound microscope is unplugged and the cover is put back on it.
Future goals: In the future labs being able to focus the microscope faster will help greatly. Something else that will be helpful is being able to identify some ciliates using the compound microscope.

 Wet Mount
Wet Mount Methyl Green Pronin Y
Methyl Green Pronin Y Methyl Cellulose
Methyl Cellulose