11/15 Lab 13: Ciliate Isolation and Characterization
Purpose
The purpose of this lab was to wrap up all the ends of the soil experiment and begin to prepare for the presentation. We continued to look for ciliates in our soil samples and took any photos that could be used in the presentation. Also we looked through our cultured plates to see if any ciliates had been obtained from last week. Before starting to observe under the microscope, we learned more about ciliate naming to understand better what we might find in the soil sample. This lab allowed us to pull all of our skills and ideas together and start on a presentation that exemplifies all of our work.
Procedure
- Obtain the soil sample from last week. It should have settled and you should be able to see three distinct layers in the order of sand, silt, clay.
- Have your lab partner hold up the tube with a ruler next to it for you to take a picture. This will allow for easier and more precise measurements.
- Now look at the tube and measure the total cm of the soil (ignore the water displaced on top). Then calculate the individual layers in cm. Now to find the percent composition of each layer, divide the individual cm by the total cm and multiply it by 100.
- Using the soil composition triangle, find where your composition puts you and record what the sample soil is.
- Now move on to the culture well you made last week. Pipette 5 drops of 5μl onto a concavity slide. Look at each drop and take pictures if any ciliates are found.
Data
Table 1~ Soil Composition
| Sample | cm | % Comp |
| Total Soil | 2.5 | n/a |
| Sand | 0.9 | 36% |
| Silt | 0.4 | 16% |
| Clay | 1.2 | 48% |
| Soil Type | Sandy Clay |
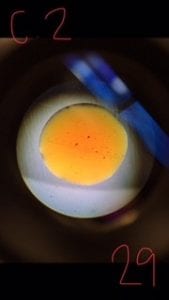
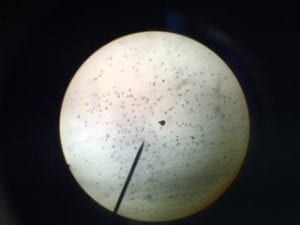
Picture of Culture Ciliates~
Storage
- The soil tube was placed back on the rack.
- The microscopes were unplugged and recovered.
- The slide was rinsed and set on a paper towel to dry.
- The culture well was covered and placed at the of the table.
- The soil plate was placed back under the fume hood.
Conclusion
This lab was a great tie up to the semester’s experiments. Since we know the lab protocol well now, we were able to work fast and efficiently. In addition, finding more ciliates allowed us to continue to practice our microscope skills and prepare for our presentation by adding more photos to what we have already collected. I am excited to present our finding and display what we have learned so far in an engaging and professional manner.