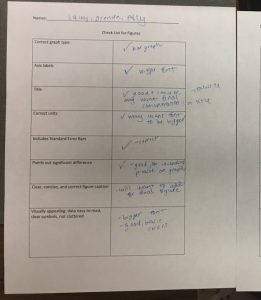
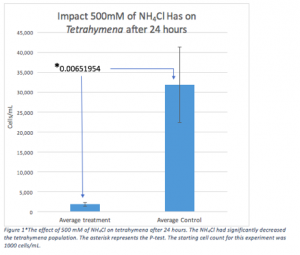
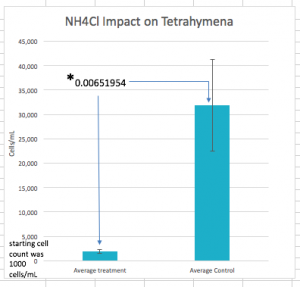
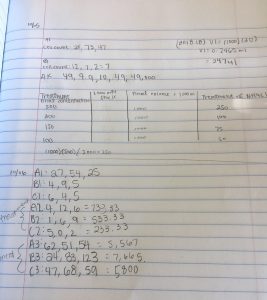
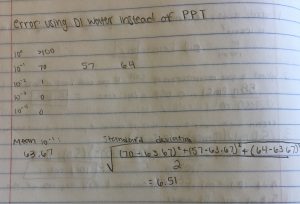Lab experiment #13 (11/16/17)
Purpose: The purpose of this experiment was to discover and try to identify the ciliates found in the previous lab.
Procedure:
- Bring the well plate with your ciliate culture to your desk.
- If you did not create a well plate because you couldn’t find ciliates in your soil sample, create a well plate using Dr. Adair’s ciliate sample.
- Examine the well plate under a dissecting microscope to find more ciliates.
- After finding ciliates in your well plate, pipette 10 microliters of the sample on to a slide.
- Use a compound microscope to find ciliates on your slide.
- Take photos of your ciliates.
- If you can’t take photos of the ciliates because they are too fast, pipette about 3 microliters of methylcellulose on your sample.
Results:
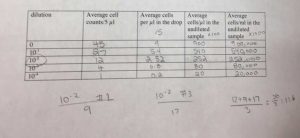
I found three ciliates in my soil sample, but I could not capture and culture them. I ended up using Dr. Adair’s sample, and I was able to find more ciliates. 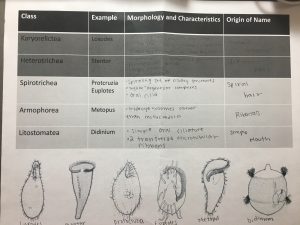
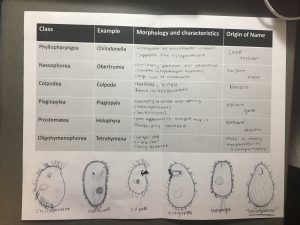

Conclusion: Overall, this experiment was an effective way to observe ciliates in different types of soil. Although I wasn’t able to capture ciliates from my soil, it was interesting to see the ciliates in Dr. Adair’s soil sample.