Lab 13: Gel Electrophoresis/ Poster Organization 4/12/18
Objective:
The objective of this lab is to perform gel electrophoresis and to observe our gel after it is imaged using BioRad technology. Another goal of today’s lab is to ensure that we start planning out how we are going to organize our poster. We also must individually brainstorm our own poster ideas then have them critiqued by our group members.
Purpose:
The purpose of this lab is to view our final gel electrophoresis attempt and to observe our results. We also will need to be able to interpret our data which we should be able to do based on previous trials. This will test your knowledge of our scientific literature and our pre-labs because that is what will determine whether or not students are able to interpret the data or not.
Procedure:
1.) Pour the 1X TAE solution into the gel powerbox.
2.) Pipet 5 micro-liters of a ladder into well one.
3.) Pipet 10 micro-liters of the -, +, and eDNA into the wells (repeat for the other group that you are sharing the gel with).
4.) Run the electrophoresis at about 100 volts for 30 minutes.
5.) While you are running the gel, use this time to discuss poster designs and the information that will go into each portion of the presentation as well as assign roles to each person in the group.
6.) After the electrophoresis, take the gel upstairs to be analyzed under UV light using the BioRad imaging technology.
7.) Review image from the BioRad technology and interpret the data and record the results for each reaction on the Excel sheet in the pre-lab module for lab 13.
***Make sure to meet up with your group members to ensure that everything gets completed on time.
Data/Observation:
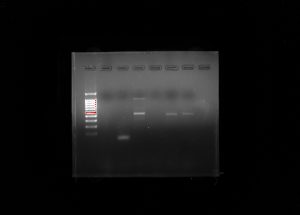
In this lab, we imaged our gel using BioRad imaging technology which clearly illustrates the bands present within the gel. Our gel showed very confusing results because we had very few bands in the positive control but we had the right band for our eDNA control which was about 420 base pairs. That is the right band for the V4 primer but it was very concerning that our positive control did show up but the environmental DNA sample did. Below is a picture of our gel that we shared with group 6. The wells go as follows: 1. Ladder, 2. Group 5 Negative Control, 3. Group 5 Positive Control, 4. Group 5 eDNA, 5. Group 6 Negative Control, 6. Positive Control, 7. Group 6 eDNA, 8. Empty. The wells 2-4 represents the results of group 5 and wells 5-7 represent group 6.
Storage:
We disposed of our gel after we were able to visualize it using the BioRad imagining technology. We did start organizing how we will format our poster project and assigned each member a task to work on. Sani: Results. Lindsay: Introduction. Kaitlyn: Abstract. Although we are starting these aspects of our poster separately, we are going to edit them together in order to make sure everyone is up to everyone’s standards. We also will make sure to contribute equally using this method. Our samples used during the gel electrophoresis were kept in case the gel electrophoresis needs to be run again and were put back a the front of the room in a tube rack.
Future Goals:
In the future, I think we should retest my groups’ positive control and the eDNA sample to clarify any discrepancies in our results. I hope that our gel electrophoresis will yield better results and allow us to submit the best samples of our controls to Illumina meta-barcoding where the DNA will be sequenced. This sequencing will allow us to further evaluate soil ciliate diversity. This could potentially lead to more taxonomic identification and for other researchers to follow in our footsteps and use a more efficient modified protocol when performing ciliate DNA extraction.