9/10/18 Soil Washing and Metadata Collection
9/10/18 Soil Washing and Metadata Collection
Objective:
The goal of this procedure was to wash and enrich the new soil that was collected previously and to collect other soil metadata. We did so that we could address our questions and begin table level study of phages in soil.
The overarching question this test seeks to address is: Is the presence of phage determined by species of oak tree from which soil was collected?
In other words, are specific oak tree species more likely to have Arthrobacter bacteria phages in the soil surrounding them?
The question specific to my lab table is: Is the a difference in the presence of phage between live oaks and red oaks on Baylor’s campus?
As a group we hope to expand our question to include more species as we gather data so that we can better address our overarching question.
Procedures and Protocols:
Materials for Aseptic zone:
- CiDecon
- 70% Ethanol
- Ethanol Burner
Materials For Soil Washing:
- Syringe filter
- .5 ml Arthrobacter
- 50 ml conical vial
- 15 ml conical vial
- LB Broth
- refrigerator
- Incubator
- Centrifuge
- Pipette
- Scale
- Weigh Boat
- Test tube stand
Materials for % Water Analysis:
- Scale
- Weigh Boat
Materials for % Sand, Silt, Clay Analysis:
- Falcon Tube
- Dispersion Fluid
- Deionized (DI) water
Materials for pH Test:
- pH vial
- DI water
- pH Paper
- pH comparison color scale
In order to complete the procedure an aseptic zone was created:
- Clean off the work space (lab table) with CiDecon applied with a squeeze bottle and wiped away with a paper towel
- Apply 70% Ethanol with a squeeze bottle, spread with a paper towel, and allow to evaporate
- Light an ethanol burner in order to use the rising heat from the flame to form the aseptic zone
*The soil washing and enrichment was conducted while other tests were being conducted simultaneously by other group members; for the sake of a simple procedural explanation, all of the steps of each of the following procedure will be detailed separately. If there was an error or if the process of one procedure effected another it will be noted*
The soil was washed and enriched according to the following procedure:
- ~2 ml of soil was placed into a 15 ml conical vial
- 10 ml of LB broth was added, bring the combined contents of the vial to the 10.5 ml mark

- The vial was shaken and vortexed intermittently for 15 minutes
- The vial was then massed and centrifuged at 10,000 g
- After centrifugation a syringe filter was to separate the lysate from the remaining sample
- ~9 ml of lysate was filtered with syringe filter into the 50 ml conical *Note this is less than the recommended 10 ml*

- ~1 ml of lysate was filtered with syringe filter into the 15 ml conical
- .5 ml of Arthrobacter was added to the 9 ml of lysate in the 50 ml conical and put in the incubator until next class ( 47 hours)
- The remaining 1 ml of lysate in the 15 ml conical was put in the fridge
% Water analysis was preformed according to the following procedure:
- A weigh boat was weighed and the mass was recorded (see table in results)
- Then a small amount of dirt was poured into the weigh boat and the combined weight was recorder (see table)
- The weigh boat was labeled with initials and date and allowed to sit in the fume hood until next lab (~48 hours)
% Sand, silt, clay analysis was preformed according to the following procedure:
- 10 ml of soil was put in a falcon tube using a tablespoon scoop

- DI water was added until the total contents of the the tube was 30 ml

- A piece of tape was attached to the tube and labeled with initials and date
- 3 drops of dispersion fluid were added to the tube
- The tube was covered with a hand and shaken for 30 seconds
- The tube was then placed under the fume hood until next lab (~48 hours)
The pH of the soil was collected according to the following procedure:
- A small amount of soil was scooped into a pH vial using a tablespoon scooper
- The rest of the vial was filled with DI wate
- The vial was shaken for 10 seconds
- Then the contents of the vial was allowed to settle for 2 minutes
- A strip of pH paper was put in the vial for 45 seconds and then compared to the pH color scale

(The image shows the resulting color change of the DI water after the pH slip was removed)
Results:
The majority of these procedures will not have results until next lab and this entry will be updated when results are available. That said the pH of the tested soil sample was between 6.0 and 6.5 leading us to estimate a pH of 6.2.
In addition the soil washing seems to have gone well and has resulted in both enriched and direct lysate for future testing.
Update:
Analysis:
These procedure were meant to learn more about our new soil samples and get us prepared for future testing. Based on our results I can assert that my soil sample is very sandy and using the chart seen below I can assert that the soil is sand or potentially sandy loam if enough material was still in suspension. I was also assert that my soil sample was slightly acidic based on the results of my pH test. This will help my group address our question further because having a collection of soil metadata will help us determine weather or not other factors besides tree species determine phage presence.
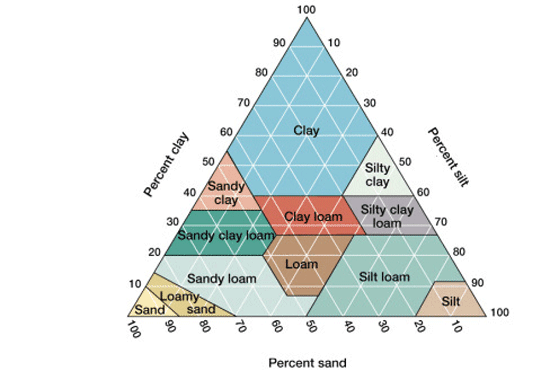 (https://samanthaapes.weebly.com/apes-in-a-box-soil-pyramid.html)
(https://samanthaapes.weebly.com/apes-in-a-box-soil-pyramid.html)
Future:
This entry has been updated to reflect the results of the metadata testing; however, when the procedures stated above were completed the initial future procedures were to simply check on the results of the experiments after the appropriate amount of time had elapsed. Now that this is completed spot tests will be preformed using the lysate created using the above procedures and depending on the results of those spot tests plaque assays or more soil collection will be preformed.


