Rationale:
Finished collecting soil metadata, such as percent water, soil texture, percent clay, percent silt, and percent sand. This metadata could possibly be used later to find correlations. A plaque assay will be run with the enriched lysate from soil C to determine whether or not there are bacteriophages in the sample that specifically target Arthrobacter.
Procedure:
- An aseptic zone was established.
- To complete the soil metadata, the “KEA 9/21/18 Soil C” weigh boat’s weight and the “KEA 9/21/18 Soil C” falcon tube different layers were recorded.
- The enriched filtered lysate from soil C was filtered with a 0.22 μL tipped syringe into a microcentrifuged tube labeled “KEA 9/24/18 FS enriched.”
- 10 mL of LB Broth, 112.5 μL of 1 M CaCl2, and 12.5 mL of 2X TA was combined into a conical vial.
- 10 μL of “KEA 9/24/18 FS enriched” was added and mixed into a test tube which already had 0.5 mL of Arthrobacter.
- This test tube then was set aside for 10 minutes, and the Top Agar mixture was placed in a hot water bath to keep it from solidifying.
- Transfer 4.5 mL of the Top Agar mixture from the conical vial onto a plate labeled “KEA 9/24/18 PA.”
- The test tube mixture was poured into the plate.
- The “KEA 9/24/18 PA” plate was placed in incubator at room temperature.
Observations:
- Dry soil appeared lighter than the wet soil. The pictures below show the soil from before and after; however, it is hard to tell color differences from the photos.

- The following calculations were performed to determine enough LB Broth, TA, and CaCl2needed for 5 plates.
|
Original Recipe
|
X5 |
|
2 mL LB Broth
|
10 mL LB Broth
|
|
2.5 mL 2X TA
|
12.5 mL 2X TA
|
| 22.5 μL CaCl2 |
112.5 μL CaCl2
|
- When the Top Agar mixture was added, bubbles formed on a few of the plates. The picture shows the bubbles.

- The falcon tube, as shown in the picture below, had a significant amount of black particles which could possibly be mulch. There is a layer of black particles then a layer of cinnamon-brown clay. The silt layer on top of these two layers appeared a whitish-gray. The remaining layer on top of this was a tannish-brown color.

Metadata:
Percent Water
Data Table
|
mass of empty weigh boat with lid (mi)
|
3.40 grams
|
|
mass of weigh boat with wet soil sample (mt)
|
6.39 grams
|
|
mass of wet soil sample (mwet soil)
mt – mi= mwet soil
|
2.99 grams
|
|
mass of weigh boat with dry soil sample (mf)
|
6.08 grams
|
|
mass of dry soil sample (mdry soil)
mf – mi= mdry soil
|
2.68 grams
|
|
mass of water in the soil sample (mwater)
mwet soil – mdry soil = mwater
|
0.31 grams
|
Equation for Percent Water


Percent Water = 10.34%
Percent Sand, Percent Silt, and Percent Clay
Data and Calculation Table
|
Texture
|
Approximate Amount |
Calculations |
Percent |
| Sand |
2.5 mL |
2.5 mL / 10 mL |
25%
|
|
Silt
|
1.0 mL |
1.0 mL / 10 mL
|
10%
|
|
Clay
|
6.5 mL |
6.5 mL / 10 mL |
65%
|
|
Total
|
10 mL |
10 mL / 10 mL |
100.00% |
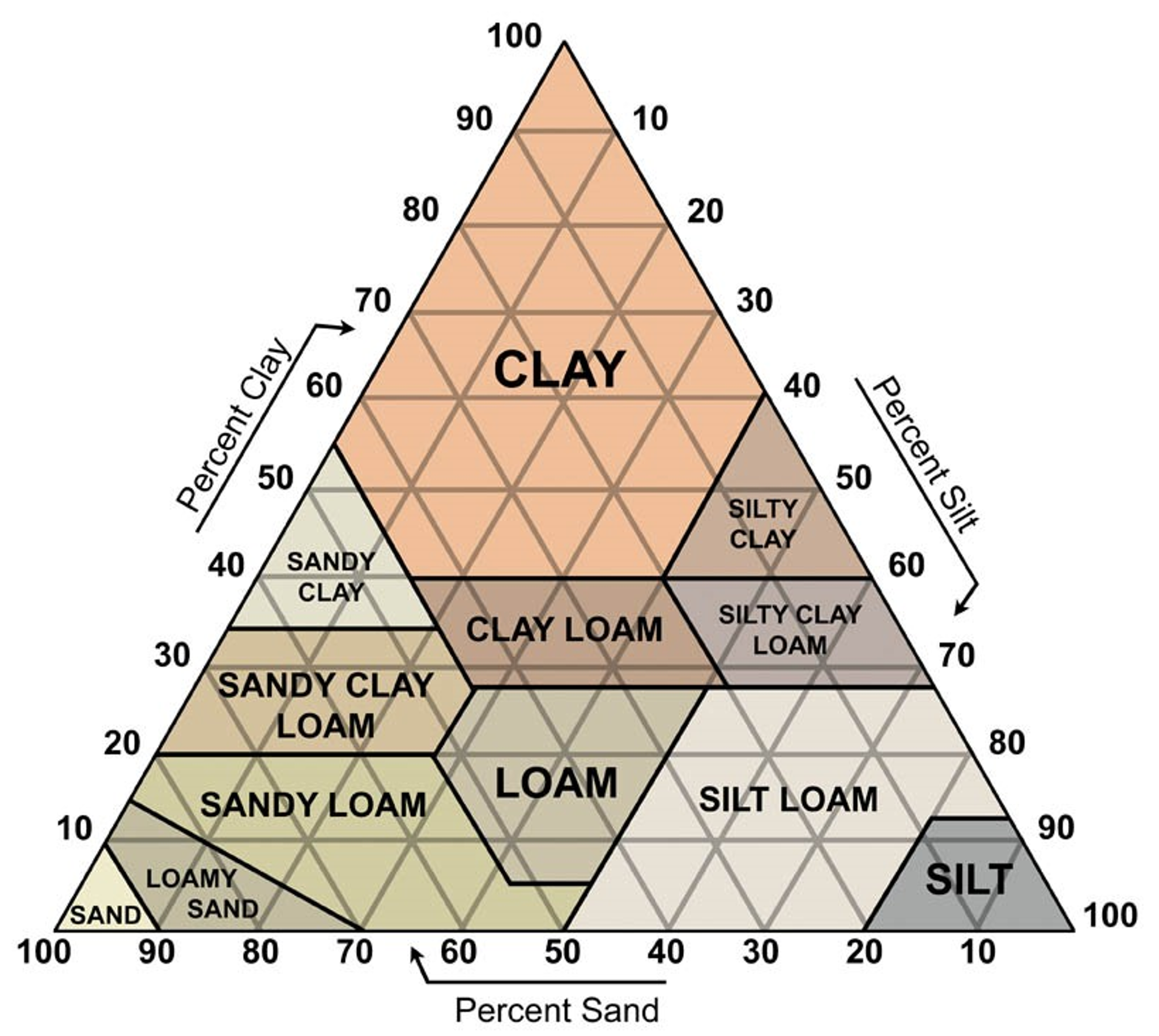
Percent Sand = 25%
Percent Silt = 10%
Percent Clay = 65%
Classifying Soil Texture
Based off the percent sand, percent silt, and percent clay, calculations using a soil texture triangle classifies the soil sample’s texture to be clay. The point is plotted on the soil texture triangle below.

This image was retrieved from the Natural Resources Conservation Service website at https://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/survey/?cid=nrcs142p2_054167.
Next Steps:
On Wednesday, if the plaque assay is contaminated, another plaque assay will be run. If the plaque assay is negative, new soil will be collected. If the plaque assay is positive, the experiment will move on to do the purification process.